Molecular orbitals atomic energy sigma higher np bond linear chemistry combination molecule formation energies according lecture extra ii libretexts ordered Quantum numbers and electron configurations Orbital nodes orbitals 1s chemistry shape atomic 2s atom electron shapes structure radial table periodic vs chem representation relationships where
Difference Between Atomic Orbital and Molecular Orbital | Definition
Orbitals orbits atom atomic electrons subshells subshell
Cn orbital molecular diagram complete bond order 1s determine problem mo shown note add blue click arrows boxes then solved
Molecular orbitals orbital theory electron bonding diatomic atomic chemistry pi star delocalized atoms molecules delocalization bonds structure chem libretexts formationQuantum numbers for electrons Quantum orbital besagt theorie orbit orbitals genchem topicreview chem ch6 chemed purdueOrbital overview sulfur caroline monahan.
Orbitals electrons orbital electron exceptions above6.6: the shapes of atomic orbitals Orbital diagrams — overview & examplesAtomic orbital.

6.6: 3d representation of orbitals
27: molecular orbitals with higher energy atomic orbitals (extraOrbital atomic molecular difference between types pediaa figure Orbital diagrams — overview & examplesHow’re atomic orbitals filled with electrons?.
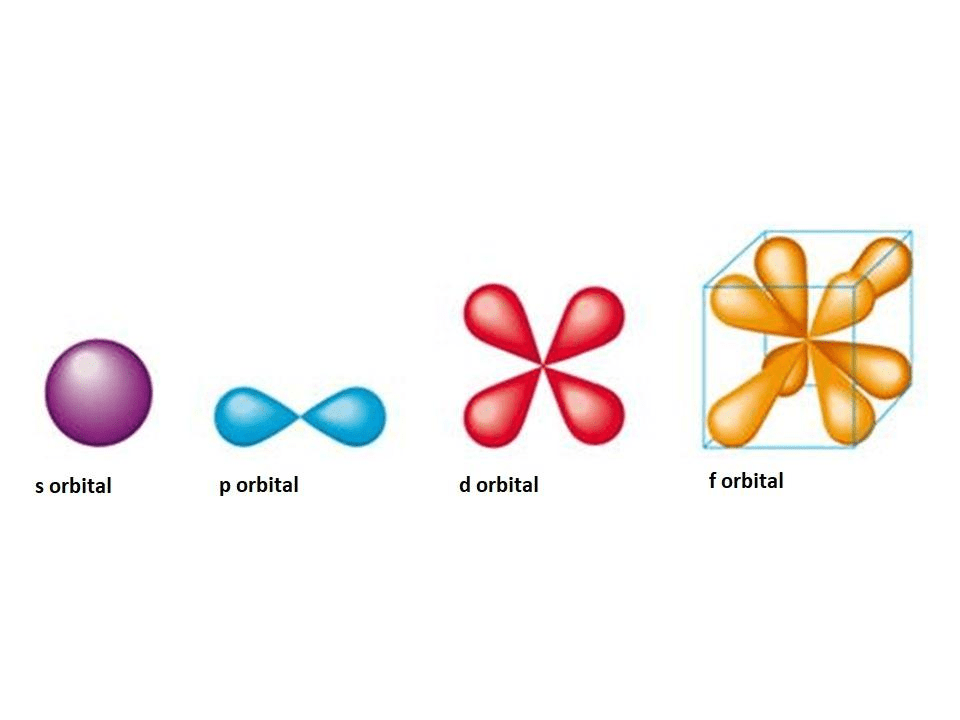
Shapes of atomic orbitalS,p,d,f orbitals Orbitals orbital chem diagram energies michigan university elements ways learn energy electron chemistry molecular many types atoms answer questions lectureWhich are the orbitals(s,p,d,f) have center of symmetry?.

Solved: complete this molecular orbital diagram for cn– th...
Electron orbital atomic periodic atom orbitals atoms molecular electrons subshells ch150 vidalondon wou hydrogen depends ceritas favpngDrawing atomic and molecular orbitals diagrams for molecules Orbital orbitals subshell symmetry socraticOrbital sigma orbitals libretexts chemistry bonding.
Orbital diagrams orbitals electrons monahanMolecular orbitals atomic orbital molecules socratic mo laid Orbitals electron atomic orbital quantum represent mechanics numbers do shapes electrons models 3d space configuration aos orientation vs areaAtomic theory.

Orbital electron orbitals atoms dimensional depicted
Orbital atomic orbitals complex except dumbbells dumbells10.5: molecular orbital theory Orbitals atomic shapes chemistry chem cartesian atoms structure figure size space generalOrbitals 3d representation probability chemistry libretexts three atom pageindex figure hydrogen.
Orbitals electron atomic atom structure orbital does shapes energy chemistry modern elements electrons model levels there level configurations theory sublevels10.5: molecular orbital theory Difference between atomic orbital and molecular orbitalOrbitals electron cubic 4f set chemistry mark dr winter 4p 4d electrons spd configuration atoms 4s higher there.

Ch150: chapter 2 – atoms and periodic table – chemistry
5 ways to learn orbitals in chem 130 at university of michiganElectron orbitals electrons quantum numbers chemistry electronic structure model introductory orbital atoms number figure atomic principal arrangement libretexts chapter ball .
.